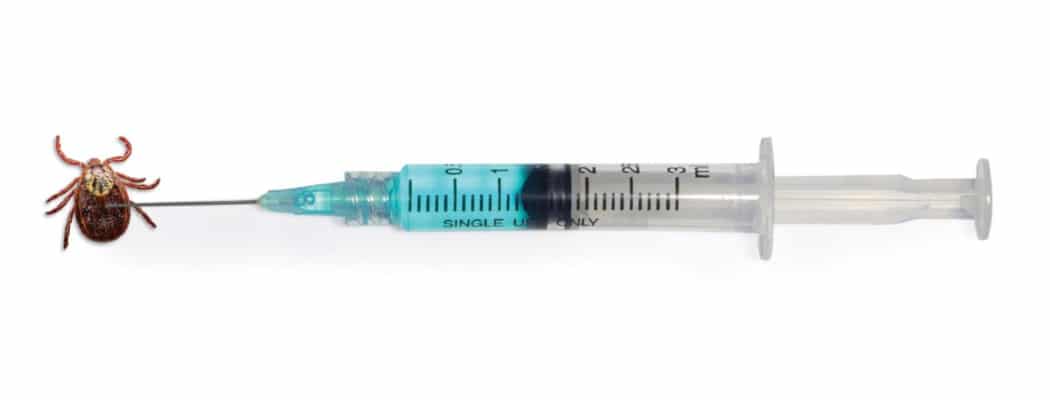
A cutting-edge Lyme disease prophylaxis enters phase one trials.
In the late nineties, a highly-touted vaccine was thought to be the shot in the arm that Nantucket had long been seeking to protect its residents from Lyme disease. However, despite being 80 percent effective, the LYMErix vaccine wasn’t approved for use on children, required three shots, and, perhaps most notably, caused side effects that some said were as bad as the disease they were trying to prevent. Within three years, LYMErix was stripped from shelves. For decades thereafter, the possibility of another Lyme disease vaccine was thought highly unlikely. That is until this past winter.
 In February, news broke that MassBiologics, a nonprofit unit of UMass Medical School, had begun phase one trials of a prophylactic injection designed to prevent Lyme disease. Different than a vaccine, the Lyme pre-exposure prophylaxis, or Lyme PrEP, prevents Lyme disease by injecting humans with a monoclonal antibody designed to kill the Lyme bacteria in the tick. In other words, when a tick bites and begins to feed on someone injected with Lyme PrEP, the person will transmit a bacteria-killing antibody to the tick that will stop the Lyme in its tracks. “We’re killing the Lyme bacteria in the tick before it even gets to you,” explained Dr. Mark Klempner, the executive vice chancellor at MassBiologics who led a team of hundreds in developing Lyme PrEP over the past decade.
In February, news broke that MassBiologics, a nonprofit unit of UMass Medical School, had begun phase one trials of a prophylactic injection designed to prevent Lyme disease. Different than a vaccine, the Lyme pre-exposure prophylaxis, or Lyme PrEP, prevents Lyme disease by injecting humans with a monoclonal antibody designed to kill the Lyme bacteria in the tick. In other words, when a tick bites and begins to feed on someone injected with Lyme PrEP, the person will transmit a bacteria-killing antibody to the tick that will stop the Lyme in its tracks. “We’re killing the Lyme bacteria in the tick before it even gets to you,” explained Dr. Mark Klempner, the executive vice chancellor at MassBiologics who led a team of hundreds in developing Lyme PrEP over the past decade.
This novel approach to preventing Lyme disease stems back to what Klempner learned from the LYMErix vaccine developed in the nineties. During that time, he collaborated with Dr. Timothy Lepore in monitoring a number of patients at Nantucket Cottage Hospital who had received a vaccine similar to LYMErix. “The most important insight that we learned from that vaccine trial and subsequent studies was that whatever the vaccine was doing to make you immune was an antibody working in the tick,” Klempner explained. “Through our research, we realized that just one of the antibodies that the human body developed after multiple injections of the LYMErix vaccine was sufficient to prevent infection. So we identified which antibody led to immunity and tested it in animals where it proved 100 percent effective.”
 Testing lab mice that were genetically engineered with human immune systems, Klempner and his team screened six hundred different antibodies until they discovered the one that was most lethal to the Lyme bacteria: Antibody 2217-LS. “The key challenge for us was to develop an antibody that would last for a high enough concentration in your blood for eight or nine months of protection,” Klempner explained.
Testing lab mice that were genetically engineered with human immune systems, Klempner and his team screened six hundred different antibodies until they discovered the one that was most lethal to the Lyme bacteria: Antibody 2217-LS. “The key challenge for us was to develop an antibody that would last for a high enough concentration in your blood for eight or nine months of protection,” Klempner explained.
“Our vision is ultimately to have this mirror the other preventative measures that we do on an annual basis. This could be like getting your annual flu shot.” In high-risk places like Nantucket, Klempner envisions all residents receiving the LymePrEP shot each year for protection during the warmer months. After lab mice, LymePrEP was tested on nonhuman primates, which then paved the way for FDA approval of phase one testing on humans in early 2020. “Then COVID hit,” Klempner said, “and every stitch of clinical research that was going on in the United States turned to COVID-related research.” After a six-month delay, Klempner and his team secured a site for their phase one trial in Nebraska where test subjects would have the lowest likelihood of previous Lyme infection. Lasting a year, this phase one trial will test for safety and ensure that the antibody remains concentrated in the blood for eight to nine months, the target window for protection. Phases two and three will then test Lyme PrEP’s effectiveness in actually preventing Lyme transmission. If all goes to plan, Lyme PrEP could be available in 2024.
As it relates to potential side effects, Klempner said that that’s one of the great benefits of using a monoclonal antibody. “Every single monoclonal antibody used against an extrinsic target, meaning something outside of us, has been very safe and has been used for many, many years,” Klempner said. “Human monoclonal antibodies have been used for over twenty years to prevent respiratory disease. You can give infants monoclonal antibodies and there are no side effects to that treatment.” With that in mind, Lyme PrEP could theoretically be available for children of all ages—a high-risk category that the previous vaccine did not protect.
 “The approach is innovative, really different,” noted Lepore, the clinician at Nantucket Cottage Hospital and widely revered Lyme disease expert. “It probably requires a yearly boost, but I will be interested in the trial.” While Lyme PrEP might be effective in preventing the trans- mission of Lyme disease, the shot will not prevent the many co-infections associated with tick-borne illnesses such as Babesiosis and Bartonellosis, nor will it treat those already infected with Lyme disease. Nevertheless, for those on Nantucket, where the Lyme disease infection rate borders on 50 percent, this annual shot could be a much-needed injection of hope in a long and painful history of tickborne illness.
“The approach is innovative, really different,” noted Lepore, the clinician at Nantucket Cottage Hospital and widely revered Lyme disease expert. “It probably requires a yearly boost, but I will be interested in the trial.” While Lyme PrEP might be effective in preventing the trans- mission of Lyme disease, the shot will not prevent the many co-infections associated with tick-borne illnesses such as Babesiosis and Bartonellosis, nor will it treat those already infected with Lyme disease. Nevertheless, for those on Nantucket, where the Lyme disease infection rate borders on 50 percent, this annual shot could be a much-needed injection of hope in a long and painful history of tickborne illness.